
Background:
Glycation and Crosslinking of Extracellular Proteins
Reactive Sugars
Sugar molecules in the blood and in the cells chemically bond to proteins and to DNA. (This bonding is called "glycation", "the Maillard reaction", "the browning reaction", or "nonenzymatic glycosylation"). This happens both inside of cells, and in the connective tissue between cells (the Extra-Cellular Matrix or ECM). There are several kinds of sugars and many kinds of proteins. Some glycation structures are good; others interfere with proper functions in the body. Inside of cells, there are protective enzymes that remove the harmful glycation structures. However, outside of cells, harmful glycation structures are not removed. This is an age-related problem in the ECM proteins, such as collagen and elastin, which are located outside of cells and provide strength and flexibility to tissues. Over time, the sugar moieties bound to the glycated proteins are chemically modified to become molecular structures called Advanced Glycation Endproducts (A.G.E.s). A.G.E.s can interfere with the proper functioning of the proteins to which they are attached. Furthermore, some of the A.G.E.s form covalent crosslinks with adjacent protein strands. This crosslinking stiffens tissues which were formerly flexible or elastic. The process happens gradually, so that crosslinks accumulate over the years on the longest-lived extracellular proteins, which do not get recycled very often. Clear evidence of this is found in the extracellular collagen and elastin. [Cerami 1987, Furber 2010]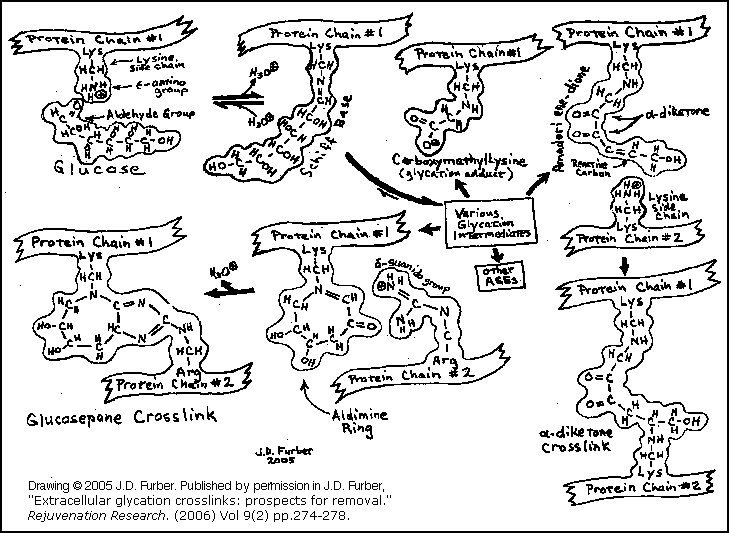
Pathological consequences
Glycation changes the shape and properties of proteins. Crosslinking reduces the flexibility, elasticity, and functionality of the proteins. Furthermore, the chemical modifications of glycation and crosslinking can initiate harmful inflammatory and autoimmune responses. "AGE and nonenzymatic crosslinks are demonstrated to signal inflammatory cytokines, extracellular matrix expansion, angiogenesis, and growth factors." [deGroof] Glycation has been found in connective tissue collagen, arterial collagen, kidney glomerular basement membrane, eye lens crystallins, nerve myelin proteins and in the circulating low-density lipoprotein (LDL) of the blood. [Bucala]Glycation and crosslinking have been implicated as strong contributors to many progressive diseases of aging, including vascular diseases (such as atherosclerosis, systolic hypertension, pulmonary hypertension, and poor capillary circulation), erectile dysfunction [Usta], kidney disease, stiffness of joints and skin, arthritis [deGroot, Verzijl], cataracts, retinopathy, neuropathy, Alzheimer's Dementia [Ulrich, Castellani], impaired wound healing, urinary incontinence, complications of diabetes, and cardiomyopathies (such as diastolic dysfunction, left ventricular hypertrophy, and congestive heart failure). [Bucala]
Arterial stiffening causes an increase in the pulse pressure wave which travels through the blood vessels with each beat of the heart. Pulse pressure is measured by subtracting diastolic blood pressure (low number) from systolic blood pressure (high number).
(P = S - D).
(For example, a patient with blood pressure of 155/90 would have a pulse pressure of 65.) Increased pulse pressure due to arterial stiffening is a leading risk factor for cardiovascular disease and brain stroke in the elderly. [Kass]
Diabetic Complications
It is significant that these same pathological processes happen at an earlier age in diabetic individuals, because their average blood sugar concentration is higher than normal. [Bucala]
Inhibitors of Glycation Crosslinking
The formation of new glycation-induced crosslinks is slowed by several drugs and natural substances: Aminoguanidine (Pimagedine) has been studied as an inhibitor of A.G.E. crosslink formation by Alteon Pharmaceuticals. (Alteon was later renamed Synvista Therapeutics. They have since gone out of business.) In human clinical trials, Pimagedine slowed the progression of diabetic kidney disease and retinopathy. Carnosine (beta-alanyl-L-histidine), a dipeptide formed naturally in human tissues, is also believed to inhibit the formation of crosslinks between proteins which have been glycated or carbonylated [Hipkiss]. Aspirin may also inhibit the formation of pathological A.G.E. crosslinks. For example, chronic users of aspirin have fewer cataracts [Bucala].
Crosslink Breakers
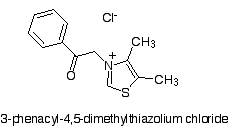
Nevertheless, aminoguanidine and aspirin do not seem to break A.G.E. crosslinks after they have formed. However, other compounds are being studied which do. Many of the known crosslink breakers are modified thiazolium salts which include an active site similar in structure to the catalytic ring of thiamine (vitamin B1). [Ulrich; Kim; Vasan; Asif] The furthest in clinical development is alagebrium chloride, which was developed by MIT and Alteon Pharmaceuticals (later re-organized and named Synvista). Alagebrium chloride was previously referred to as ALT-711.It's chemical name is 4,5-dimethyl-3-(2-oxo-2-phenylethyl)-thiazolium chloride (PTC).
The molecular structure is shown below (CAS number 341028-37-3) (ChemSpider ID: 187495):
Published Animal Tests
Early testing of alagebrium in animals showed such promising results, that the FDA gave the green light to starting human clinical trials.One early trial took crosslinked collagen fiber from the tails of old rats. After soaking them for a short time in a dilute solution of alagebrium in water, then pulling or stretching the collagen strands show greater elongation and flexibility. [Vasan 1996; Ulrich 1997]
Another test took elderly dogs that were suffering from enlarged, stiff heart-wall muscles. This is a condition that causes high blood pressure and heart failure. These dogs were given alagebrium in their drinking water. After only 30 days, their hearts had rejuvenated to the conditions of younger, healthier dogs. [Asif 2000]
Published Human Clinical Trials
Alagebrium chloride (oral) has been tested in several human clinical trials in the United States. [Melton, deGroof] During the year 2000, in earlier phase 2a clinical trials, alagebrium demonstrated the ability to improve the flexibility of arteries, and to reduce arterial pulse pressure. In this double-blind trial, 62 people took a dosage of 210 mg per day for two months, while 31 other people received placebo. [Kass] In the "Diamond" clinical trial for diastolic heart failure, beginning in mid-2002, 20 people took 420 mg per day in 2 doses of 210 mg. By February 2005, over 1100 people had taken alagebrium (or PTC) in various clinical trials.
Sprague-Dawley Rats cause temporary concern
So far, the safety profile of the drug appears to be excellent in human subjects. However, a poor choice of lab rat species for a long-term feeding test caused confusing results. In December 2004, a study feeding alagebrium to lab rats for two years found an increased rate of liver cell alterations in male rats, but not the females. This strain of lab rat (Sprague-Dawley) often develops liver cell alterations spontaneously, even when not given any experimental drugs. The rate is comparable to effects caused in Sprague-Dawley rats by other, already approved drugs, such as the statins. So seeing these abnormalities in Sprague-Dawley rats does not necessarily indicate a problem for humans. Following further study, FDA allowed clinical trials to proceed.No Harmful Interactions
No harmful interactions of alagebium with other drugs have been observed in the human clinical trials. Any subjects who had been already taking other blood pressure medications continued on their previous medications, in addition to taking alagebrium. Benefits of alagebrium were observed in addition to any benefits from the other blood pressure medications.In December 2004, Synvista Therapeutics (Alteon) announced a phase 2 clinical trial of alagebrium to reverse erectile dysfunction. Subjects were slated to be taking 200 mg, once per day. In 2009, Synvista ran out of money and went out of business, without completing its clinical trials for alagebrium. ClinicalTrials.gov
Benefits from taking ECM Crosslink-Breakers
After pathological extracellular crosslinks get broken, the body functions better. Age-related stiffness in many organ systems is reversed, so they function better. Chronic inflammation due to A.G.E.s is reduced. Combining the formal reports from the clinical trials and informal interviews with several dozen experimental subjects gives this list of reported benefits from taking alagebrium orally:- Lowering systolic blood pressure and pulse pressure, if they were too high.
- Partial reversal of pathological heart enlargement and stiffening associated with DHF (diastolic heart failure).
- Increased flexibility of large muscles when performing stretching exercises.
- Reduced or eliminated joint pain when walking or exercising.
- Reduced or eliminated peripheral neuropathy (tingling in the fingers or toes). This might be due to improved capillary circulation better nourishing the peripheral nerves. Perhaps the nerve sheath is being made more flexible so that it no longer squeezes the nerves. Or it could be due to improvements in inflammation or redox status.
- Improved bladder elasticity: Several senior subjects reported that they could hold longer and more easily before needing to urinate. They noticed that they did not need to get up as often in the middle of the night to urinate, and that they could sit through long meetings and car trips. In a young person, the walls of the bladder are elastic, so the bladder expands as it fills with urine. Age-related crosslinking stiffens the bladder walls, reducing its capacity to expand, so many older people notice that they need to urinate more frequently than they did when they were young. The alagebrium crosslink breaker appears to restore flexibility and bladder capacity.
- Improved erectile function.
- Improved kidney function in cases of diabetic or age-related nephropathy (preclinical animal studies). [Forbes]
Sources of Experimental Materials
Experimenters wishing to work with alagebrium may contact Legendary Pharmaceuticals to enquire regarding how to obtain the purified material. info@LegendaryPharma.com Alagebrium can be supplied in vegetarian capsules of 100 mg or 200 mg. It is also available in a one-ounce dropper bottle (30 mL of 100 mg/mL solution in purified water). It can also be supplied as a pure powder. This material is produced in a commercial lab according to current good manufacturing practices (cGMP). Please be aware that Legendary Pharmaceuticals is not a pharmacy, and that this experimental material has not completed the drug approval process by the FDA.Future Research Directions
Thiazolium breakers, such as alagebrium, break alpha-diketone crosslinks, and have proven beneficial in early human clinical trials, as well as in animal testing. However, during aging or diabetes, several kinds of chemical crosslinks slowly accumulate as a result of glycation. Crosslinks other than alpha-diketone, mainly glucosepane, are not broken by thiazolium compounds. We would like to discover new drugs, able to break glucosepane crosslinks. These could be used in addition to thiazolium breakers. The combination could break all of the major pathological glycation crosslinks which accumulate during aging or with diabetes. The results could be much more extensive restoration of tissue elasticity than that provided by thiazolium breakers alone.Reading and Reference
Overview and Summary Articles:
- Melton L. "AGE Breakers," Scientific American. p. 16 (July 2000).
- Borek C. "AGE Breakers," Life Extension Magazine. (Life Extension Foundation, Aug 2001).
- Cerami A. "Pharmaceutical intervention of advanced glycation endproducts", in Ageing vulnerability: causes and interventions. Novartis Bulletin. Symposium 235 (2000).
- Melton L. AGEing: Breaking the bonds, Novartis Bulletin. (2000).
Scientific Reviews:
- Furber JD. "Repairing Extracellular Aging and Glycation,"
Chapter 19 in
The Future of Aging: Pathways to Human Life ExtensionEd. by G.M. Fahy, et.al.(Springer Science+Business Media B.V. 2010) DOI 10.1007/978-90-481-3999-6_19.
Author's full text uncorrected proof with figures: PDF - Furber JD. "Extracellular glycation crosslinks: prospects for removal."
Rejuvenation Research. (2006 Summer); Vol. 9(2): pp.274-278. PMID: 16706655. Publisher's web site Mary Ann Liebert.
Full text PDF from the author. - deGroof RC. " Remodeling of Age- and Diabetes-Related Changes in Extracellular Matrix", Proceedings of 10th International Association of Biomedical Gerontology. New York Academy of Sciences. (2003).
- Cerami A, Vlassara H, Brownlee M. "Glucose and Aging", Scientific American. 256: 90 - 96 (1987).
- Bucala R, Cerami A. "Advanced Glycosylation: Chemistry, Biology, and Implications for Diabetes and Aging," Advances in Pharmacology, Volume 23. (1992) pp. 1 - 34.
- Verzijl N, Bank RA, TeKoppele JM, DeGroot J. "AGEing and Osteoarthritis: A Different Perspective," Current Opinion in Rheumatology (2003 Sep) 15(5):616-22. [view Abstract]
Research Articles:
- Kim T, Spiegel DA. "The Unique Reactivity of N-Phenacyl-Derived Thiazolium Salts Toward alpha-Dicarbonyl Compounds." Rejuvenation Research 2013 Feb;16(1):43-50. doi: 10.1089/rej.2012.1370. PubMed PMID: 23186164.
- Kass DA, Shapiro EP, M Kawaguchi, A R Capriotti, A Scuteri, R C deGroof, E G Lakatta. "Improved Arterial Compliance by a Novel Advanced Glycation End-Product Crosslink Breaker", Circulation (September 28, 2001) (American Heart Association: Rapid Track published online before print, September 4, 2001, 10.1161/hc3801.097806.)
- P Ulrich, X Zhang. "Pharmacological reversal of advanced glycation end-product-mediated protein crosslinking," Diabetologia. 40: S157-S159 (1997).
- S Vasan, Xin Zhang, Xini Zhang, A Kapurniotu, J Bernhagen, S Telchberg, J Basgen, D Wagle, D Shih, I Terlecky, R Bucala, A Cerami, J Egan, P Ulrich. "An agent cleaving glucose-derived protein crosslinks in vitro and in vivo," Nature. 382: 275 - 278 (18 July 1996).
- Castellani RJ, Harris PL, Sayre LM, Fujii J, Taniguchi N, Vitek MP, Founds H, Atwood CS, Perry G, Smith MA. "Active Glycation in Neurofibrillary pathology of Alzheimer's Disease: N(epsilon)-(Carboxymethyl) Lysine and Hexitol-Lysine", Free Radical Biology & Medicine Vol. 31 (2), pp. 175-180, (2001).
- M Asif, J Egan, S Vasan, G N Jyothirmayi, MR Masurekar, S Lopez, C Williams, R L Torres, D Wagle, P Ulrich, A Cerami, M Brines, T J Regan. "An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness," PNAS. 97(6): 2809-2813 (14 March 2000).
- M F Usta, M Kendirci, T J Bivalacqua, S Gur, W J G Hellstrom, N A Foxwell, S Cellek. "Delayed Administration of ALT-711, but not of Aminoguanidine, Improves Erectile Function in Streptozotocin Diabetic Rats: Curative Versus Preventive Medicine," 11th World Congress of the International Society for Sexual and Impotence Research, Buenos Aires (October 2004). [news release]
- deGroot J, et.al. "Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis," Arthritis & Rheumatism. (Published Online: 5 Apr 2004) Volume 50(4): 1207 - 1215. [view Abstract]
- Forbes JM, et.al. "The breakdown of pre-existing advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes," The FASEB Journal express article 10.1096/fj.02-1102fje. (Published online: July 18, 2003)
- Hipkiss AR, Brownson C, Carrier MJ. "Carnosine, the anti-ageing, anti-oxidant dipeptide, may react with protein carbonyl groups," Mech Ageing Dev. 2001 Sep 15;122(13):1431-45.
Hot Topics Navigation
[Network Flow Chart - Systems Biology of Aging]
[Extracellular Glycation and Crosslinks]
[Research Program] [Scientific Advisory Board] [Contact Information]
[ Link to ==> Amazon.com ]
Legendary Pharmaceuticals is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to amazon.com.
Rev. 7 December 2024. © 2000 - 2024 by John D. Furber. All rights Reserved.